Formulation Capabilities
GMP Manufacturing
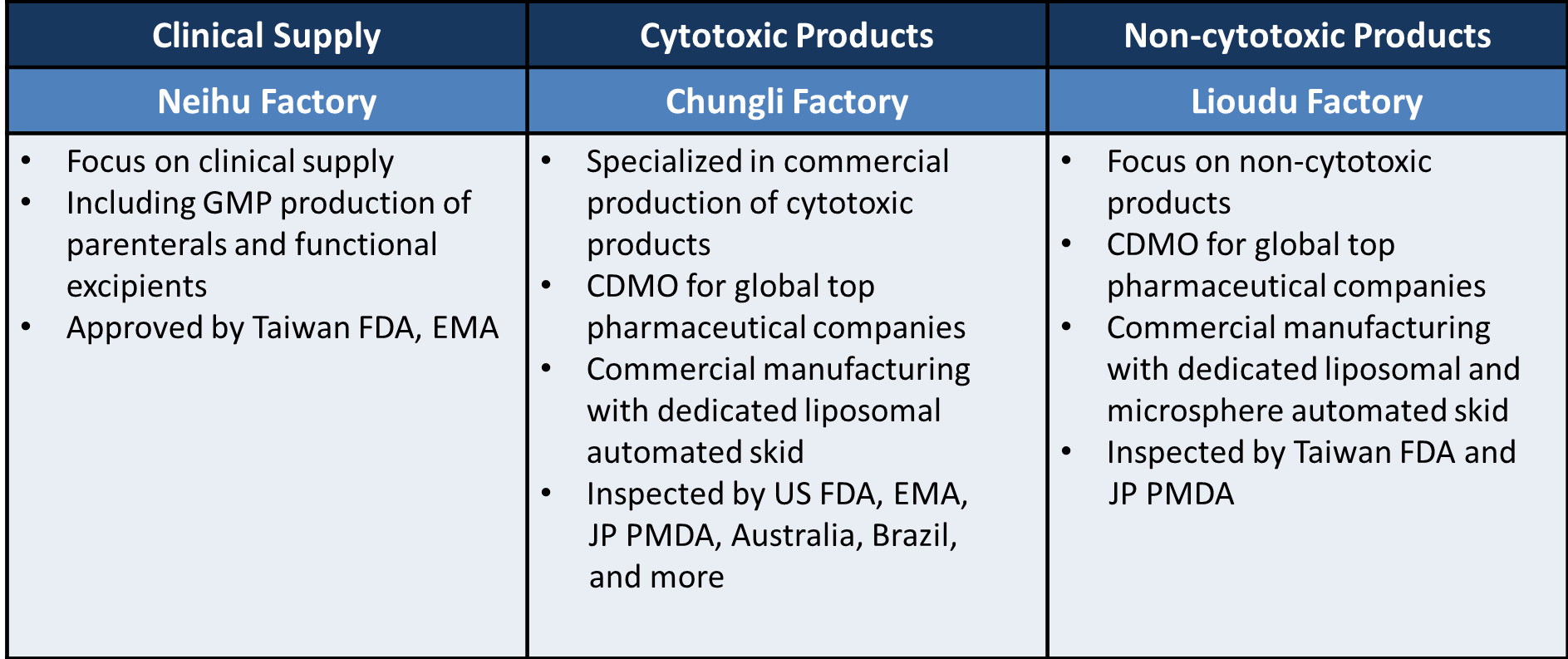
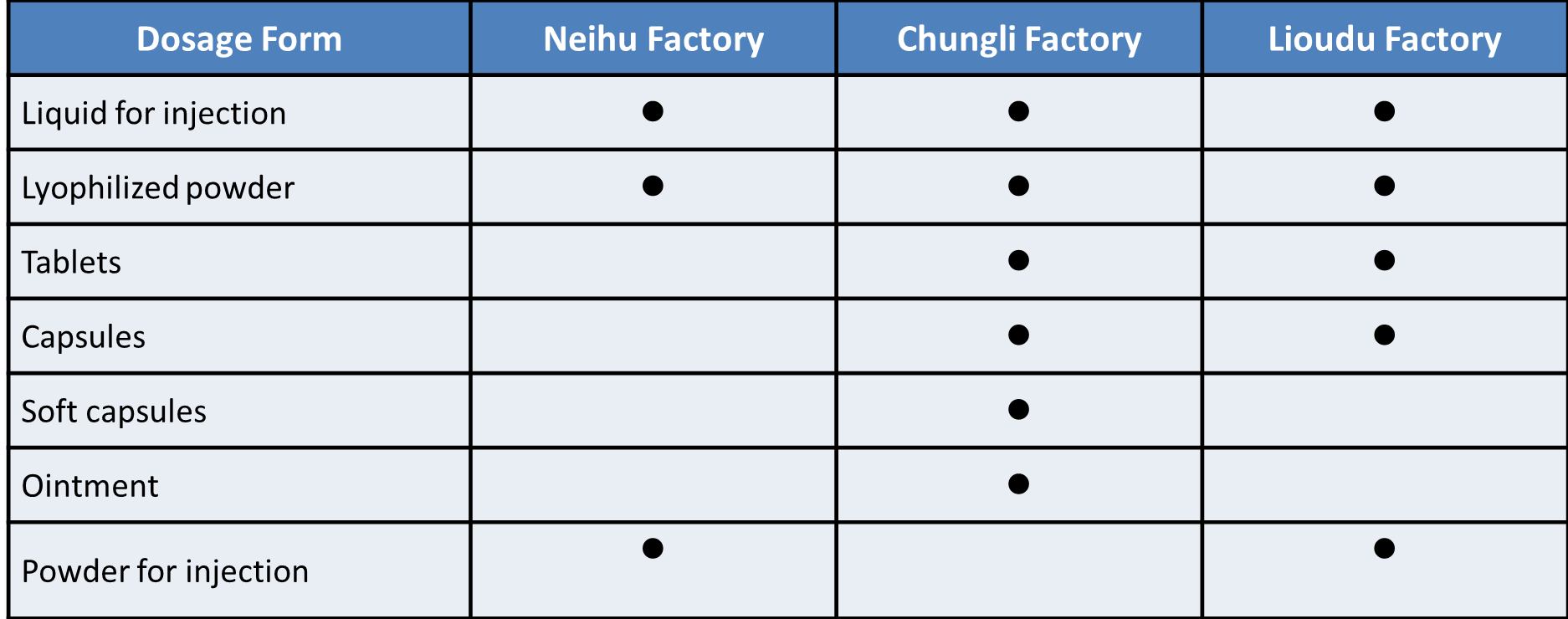
TTY has three cGMP manufacturing sites in Taiwan, which are located in Chungli, Lioudu and Neihu. Each focuses on production of cytotoxic drugs, non-cytotoxic drugs, and clinical supply. Our facilities have been inspected by numerous regulatory agencies (US FDA, EU EMA, Japan PMDA, Taiwan FDA) as well as reputable partners all over the world. We have the capability to cover all phases of drug development, clinical trial material manufacturing, commercial production and packaging. Our extensive experience and technical knowledge enables us to provide robust, efficient and flexible production.