Formulation Capabilities
Analytical Development
Ensure Quality and Stability Throughout the Development Process
Our experienced analytical specialists work alongside our formulation and manufacturing teams, providing expert advice and an enormous breadth of advanced analytical technologies.
Our experienced analytical specialists work alongside our formulation and manufacturing teams, providing expert advice and an enormous breadth of advanced analytical technologies.
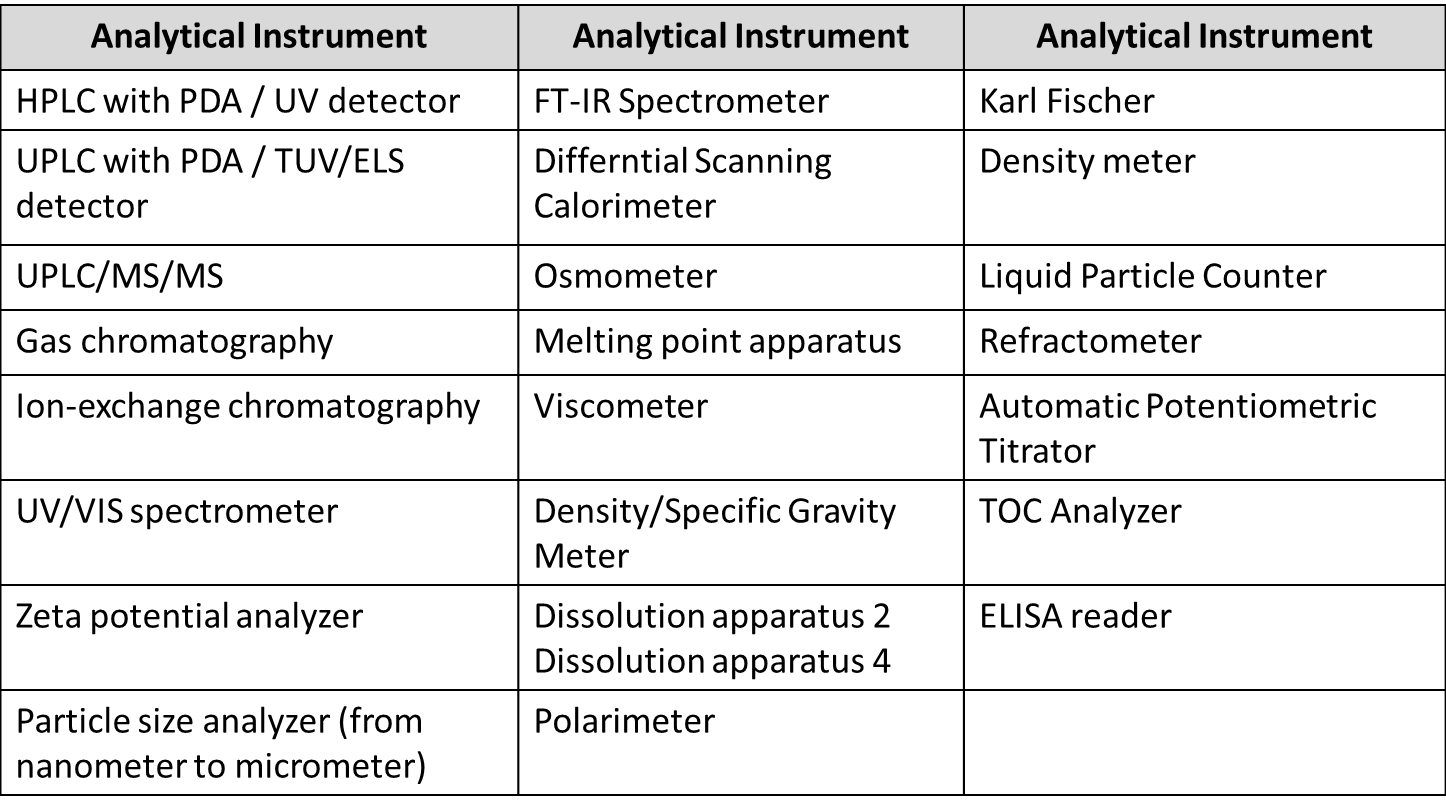
Our analytical and quality control services include:
- Analytical method development, validation and transfer
- Stability-indicating assays using UPLC and HPLC (including forced degradation studies)
- Formal and informal stability studies
- Batch release and raw material method development
- Chemical and physical testing
- In vitro release profiling
- Drug substance characterization
- The identification and quantification of impurities

Microbiological Development
TTY offers a broad range of compliant, high quality microbiological testing. Tests are performed according to official compendial methods such as USP, EP, BP, JP, AAMI, ISO standards and client specific protocols.
TTY offers a broad range of compliant, high quality microbiological testing. Tests are performed according to official compendial methods such as USP, EP, BP, JP, AAMI, ISO standards and client specific protocols.
COMPENDIAL SUPPORT:


- Microbial limits testing based on USP, EP, JP
- LAL endotoxin quantification (gel-clot, kinetic chromogenic methods)
- Bioburden testing
- Sterility testing
- Antimicrobial effectiveness testing
- Container/Closure Integrity testing (microbial ingression)
- Water quality testing
- Environmental monitoring
- Specialized microbiology testing