Lioudu Factory
Features
Lioudu factory was established in 2011. Previously, it was Taiwan Shionogi Lioudu factory. In 2011, a renovation plan for oral administration plant was initiated. In 2012, an international standard injection plant was built to enhance medicine manufacturing quality and to connect with international market.
Lioudu factory was established in 2011. Previously, it was Taiwan Shionogi Lioudu factory. In 2011, a renovation plan for oral administration plant was initiated. In 2012, an international standard injection plant was built to enhance medicine manufacturing quality and to connect with international market.
- Major Preparations Manufactured: non-cytotoxic oral tablet, capsule, liquid injection and lyophilized liposome, as well as steriled preparation and filling of bio-drugs (vaccines).
- Oral preparation plant and injection preparation plant complied with PIC/S GMP International requirements.
- Injection preparation plant is equipped with Italian automatic tunnel-styled filling line(vial washing machine, depyrogenating tunnel, filling machine, lyophilizer and capping machine) together with aseptic preparation manufacturing plant and advanced equipment. Safety protection for operating personnel is established as basic requirement.
- Liposome preparation manufacturing center.
- International medicine manufacturing standards passing factory inspections by Taiwan TFDA and Japan PMDA. Applications for international inspections are passed by U.S. and Europe.
- The best collaboration partner for international pharmaceutical factory CMO (Contract Manufacturing Organization).
- Capability to develop new product microbiology analysis methods consistent with USP, EP, JP Pharmacopoeia, PIC/S GMP and requirements from world advanced countries.

Capacity
- Oral Tablet: 350M Tablets/Year
- Oral Capsule: 72M Capsules/Year
- Liquid Injection: 1.5M Vials/Year
- Lyophilized Liposome: 200K Vials/Year
Important Equipments



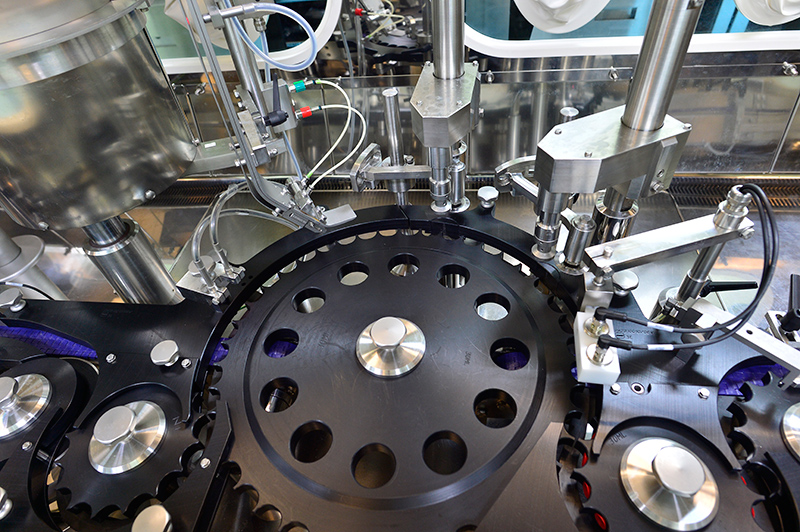
- Italy Automatic Tunnel-Styled Filling Machine (vial washing machine, depyrogenating tunnel, filling machine, lyophilizer and capping machine)
- GE Kaye Validator
- Water System Equipment from European Manufacturer
- GE On Line TOC (Total Organic Carbon) Analyzer
- Japan Automatic Compress machine
- Japan Automatic Capsule Filling Machine
- Italy Automatic Capsule Filling Machine
- Germany Bacteria Identification Instrument Capable of Accurate Identification of “Species”
- Endotoxin Detector Capable of Detecting 0.005EU/ml Extreme Low Amount of Endotoxin
- TOC (Total Organic Carbon) Analyzer
- Waters HPLC and UPLC Equipment
- Malvern Particle Size Analyzer
- TA DSC (Differential Scanning Calorimeters)
- Fluorometer




GMP Official Certification
Oral Administration Factory
2024 Taiwan Official PIC/S GMP Inspection
2022 Taiwan Official PIC/S GMP Inspection
2022 Japan Health Authorities by Written Review
2020 Taiwan Official PIC/S GMP Inspection
2018 Taiwan Official PIC/S GMP Inspection
2016 Japan Health Authorities Inspection
2016 Taiwan Official PIC/S GMP Inspection
2013 Taiwan Official PIC/S GMP Inspection
Oral Administration Factory
2024 Taiwan Official PIC/S GMP Inspection
2022 Taiwan Official PIC/S GMP Inspection
2022 Japan Health Authorities by Written Review
2020 Taiwan Official PIC/S GMP Inspection
2018 Taiwan Official PIC/S GMP Inspection
2016 Japan Health Authorities Inspection
2016 Taiwan Official PIC/S GMP Inspection
2013 Taiwan Official PIC/S GMP Inspection
Injection Preparation Factory
2024 Taiwan Official PIC/S GMP Inspection
2024 EMA (FIMEA Finland) GMP Inspection
2022 Australia Health Authorities Inspection
2022 Taiwan Official PIC/S GMP Inspection
2022 US FDA Inspection
2021 Taiwan Official PIC/S GMP Inspection
2020 Taiwan Official PIC/S GMP Inspection
2018 Taiwan Official PIC/S GMP Inspection
2016 Taiwan Official PIC/S GMP Inspection
2024 Taiwan Official PIC/S GMP Inspection
2024 EMA (FIMEA Finland) GMP Inspection
2022 Australia Health Authorities Inspection
2022 Taiwan Official PIC/S GMP Inspection
2022 US FDA Inspection
2021 Taiwan Official PIC/S GMP Inspection
2020 Taiwan Official PIC/S GMP Inspection
2018 Taiwan Official PIC/S GMP Inspection
2016 Taiwan Official PIC/S GMP Inspection
Powder Filling Factory
2024 Taiwan Official PIC/S GMP Inspection
2022 Taiwan Official PIC/S GMP Inspection
2024 Taiwan Official PIC/S GMP Inspection
2022 Taiwan Official PIC/S GMP Inspection
Certificate of Taiwan GMP


Certification as a Foreign Manufacturer of Japanese Pharmaceuticals


Australia Certificate of GMP Compliance of a Manufacturer



EMA (FIMEA Finland) GMP Certificate


