Chungli Factory
Features
TTY Biopharm Company Limited Chungli Factory was established in Chungli Industrial Park in 1968. To meet with international drug manufacturing standards and to connect with international markets, the original oncology injection plant and oncology oral drug plant had been re-designed in 2001 and 2007 respectively in order to enhance its sterile operations, quality system, moving lines and exclusive equipment requirements, and establish itself as a professional manufacturer for cancer treatment consistent with international PIC/S GMP requirements. It has become the first enterprise to have an independent cancer injection factory.
Major Dosage manufactured: cytotoxicity oncology injection, liposome injection and oncology oral capsule.
Its features include:
TTY Biopharm Company Limited Chungli Factory was established in Chungli Industrial Park in 1968. To meet with international drug manufacturing standards and to connect with international markets, the original oncology injection plant and oncology oral drug plant had been re-designed in 2001 and 2007 respectively in order to enhance its sterile operations, quality system, moving lines and exclusive equipment requirements, and establish itself as a professional manufacturer for cancer treatment consistent with international PIC/S GMP requirements. It has become the first enterprise to have an independent cancer injection factory.
Major Dosage manufactured: cytotoxicity oncology injection, liposome injection and oncology oral capsule.
Its features include:
- The only liposome automatic manufacturing system in Taiwan.
- Oncology facilities and exclusive equipment comply with PICS/GMP International requirements.
- International drug manufacturing standards passing various inspections conducted by countries of U.S., Europe and Japan with products already marketed globally.
- The best collaboration partner for international pharmaceutical factory CMO (Contract Manufacturing Organization).

Capacity
- Liposome Injection: 600K – 700K Vials/Year
- Cytotoxicity Oncology Injection: 2.5M Vials/Year
- Oncology Oral Capsule 25M Capsules/Year
GMP Official Certifications
- 2024 Passed EMA (Finland) Inspection; passed Taiwan Official PIC/S GMP Inspections
- 2023 Passed Taiwan Official PIC/S GMP Inspection
- 2021 Passed Taiwan Official PIC/S GMP Inspection; passed Japan Health Authorities by Written Review
- 2019 Passed Taiwan Official PIC/S GMP Inspection
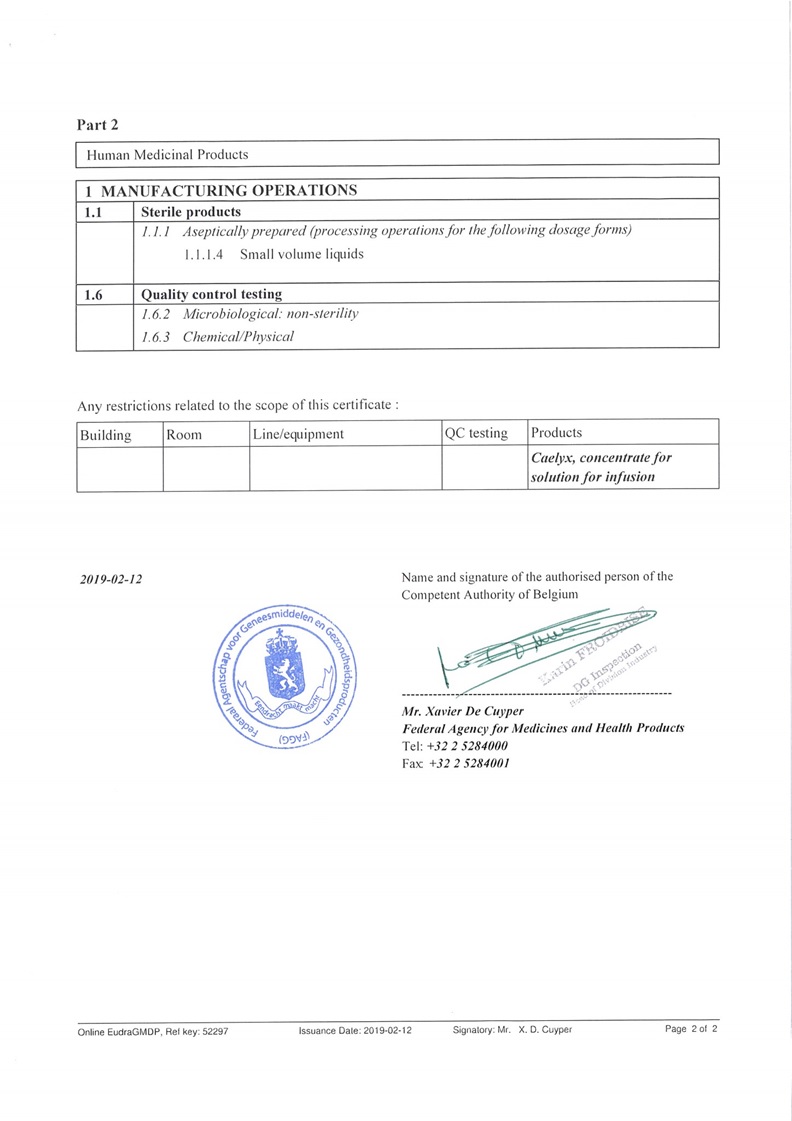
- 2018 Passed Kenya, U.S. FDA, EMA (Belgium) and Korea Health Authorities’ Inspections
- 2017 Passed Belarus and Indonesia Health Authorities’ Inspections
- 2016 Passed U.S. FDA Inspection
- 2015 Passed Korea, Kazakhstan and EMA (Belgium) Health Authorities’ Inspections
- 2014 Passed EMA (Belgium) and Turkey Health Authorities’ Inspections
- 2013 Passed U.S. FDA and Japan Health Authorities’ Inspections
- 2012 Passed Japan Health Authorities’ Inspection
- 2011 Passed EMA (Germany) and Brazil Health Authorities’ Inspections
- 2010 Passed Jordan Inspection
- 2009 Passed EMA (Hungary) Inspection
- 2008 Passed EMA (Hungary) and Arab Health Authorities’ Inspections
U.S. FDA EIR Report


Certificate of Brazil


Official Documentation of Passed Belgium Inspection



Certificate of Turkey




Translation of Passed Belarus Inspection




Certificate of Japan


Certificate of TFDA PIC/S GMP


Certificate of GMP Compliance issued by Finland

