About TTY
TTY Biopharm Company Limited was established in 1960. For more than fifty years, TTY Biopharm has transformed from a production and sales oriented traditional generic drug pharmaceutical factory into development and marketing of branded generic drugs. With unending self-renovation and transformation, we utilize the experience, technology, and relationship network accumulated over the decades to seek improvement. Nowadays, we redefine ourselves as a biotech company focusing on developing special formulations and new drugs. We hope to achieve our enterprise vision of “improving the quality of human life with scientific innovation.”
Vision: To improve the quality of human life with scientific innovation.
Mission:
- Commitment to the development and manufacture of pharmaceuticals with special formulations (patentable or high barrier products) and biopharmaceuticals and new drugs to perfect TTY product mixes; continued improvement and research of a high-barrier formulation/pharmaceutical development platform and constant extension of usage benefits.
- The company is dedicated to being specialized in international development of the anti-cancer field as well as continued cultivation of development and marketing for critical diseases and anti-infection drugs and vaccines. Additionally, the company also aggressively invests in healthcare products in attempting to become a comprehensive international pharmaceutical company.
- The company aims to be one of the most innovative biopharmaceutical manufacturers in the world and an ideal cooperation partner of international biotech companies in the field of pharmaceutical development and international marketing.
TTY Culture:
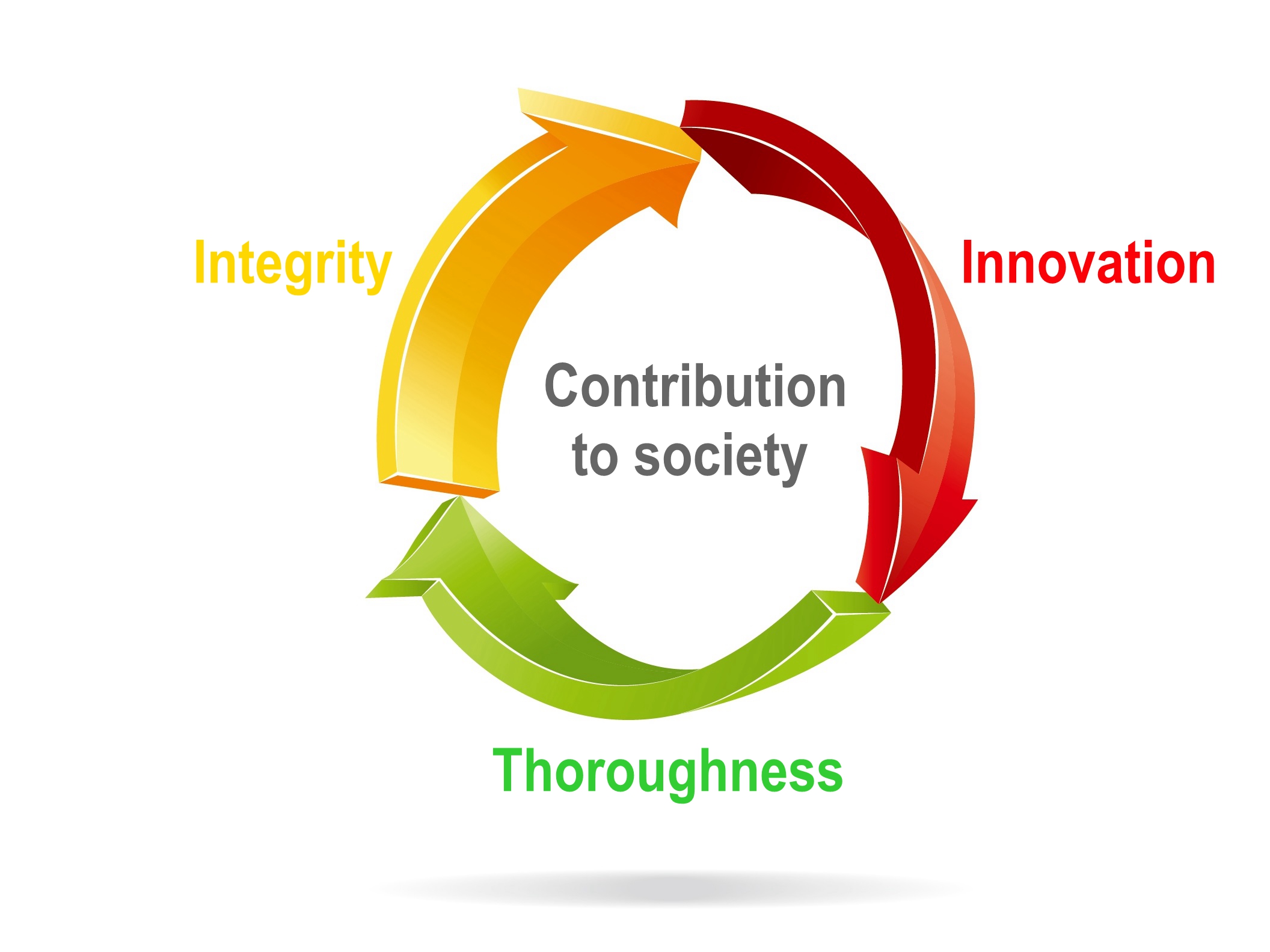
The Enterprise Culture of TTY is to conduct everything with integrity. We start from the solid basics, and encourage innovation and thorough execution. More importantly, while creating maximum benefit for the organization, we also engage in learning, growth, and we wish to become benevolent social citizens and offer contribution with every member of the big family of TTY!
The Enterprise Culture of TTY is to conduct everything with integrity. We start from the solid basics, and encourage innovation and thorough execution. More importantly, while creating maximum benefit for the organization, we also engage in learning, growth, and we wish to become benevolent social citizens and offer contribution with every member of the big family of TTY!
We deeply believe that “people” is the most important core and asset for an organization. With this, under a complete corporation organization structure, we provide resources needed for respective business groups in order to develop synergy accordingly. We have a stable and pragmatic operation management team, efficient logistic support and a solid business marketing team, all of which have enabled TTY business to develop amidst stability.
There are ascribed to our important business units and core competencies:
- TTY Oncology Business Unit (TOT), TTY Intensive Care Business Unit (TIT), and Key Account Management (KAM) are TTY’s three major product axles. They not only generate stable profits for the company, but they also explore development opportunities overseas aggressively in a bid to expand TTY’s global territory and composition geographically.
- Neihu Pharmaceutical Development team has world-class of two major technology platforms of liposome and microsphere, which cannot be replaced or duplicated.
- Two major manufacturing centers located in Chungli and Lioudu are our production advantages. They’re not only comply with Taiwan PIC/S GMP manufacturing regulations but also receive factory inspection certifications from multiple countries around the world.
- The Project & Regulatory Affairs Division is equipped with analysis capability to verify acquisition of subsequent drug permit certificate when there’s new product preliminary assessment. It provides assistance for product material preparation during domestic and overseas inspection and registration, and plays a key role in consultation when delivering industry requirements for domestic governments. This has further enhanced Taiwan’s international competitiveness.
- The Legal and Compliance Division provides risk prevention to the Group’s overall legal risks and supports contract review and legal opinion consultation for the Group and its affiliated enterprises. It provides various legal opinions and related risk assessments to major investments of mergers and acquisitions or all litigations related to the Group. In the meantime, it also provides professional assessments on intellectual property protection and market risks for the company’s product portfolio, and eliminates intellectual property obstacles encountered for issues of licensing, transaction and litigation, and improve product development speed and success rate and reduce launch risk.
- The Business Development Devision is responsible for establishing overseas subsidiaries as well as business scopes for strategic alliance, innovation business, collaboration management and mergers and acquisitions strategy.
Under our well rounded talent development mechanisms in TTY, we encourage lifetime learning, knowledge sharing, driving to one’s own excellence, or even proactively volunteering for tasks with developmental potentials to enhance personal capability. On one hand, through the knowledge and learning platform of TTY University, we help our people think systematically, understand our strengths, learn about industrial value chain knowledge, and transform knowledge into capabilities. On the other hand, through continuous PDP (Personal Development Program), we provide well-timed advices on work or developmental recommendations. We hope that every one of us can subscribe and enforce the core values of TTY, and seek to internalize the core values, and to expand and maximize both the capability of our people and contribution of our company.
TTY is committed to building itself as a biotech pharmaceutical company advanced in drug development and international marketing. On one hand, TTY is continuously improving its ability to gear to international developments in areas such as international technical documentation formats (CMC, CTD), execution of international clinical trials, and manufacturing process which matches EMEA and FDA specifications. On the other hand, TTY continues to carefully select the most suitable collaborative partners for new drug development around the world and various target markets. Other than continuing operation in the core channels (medical centers, regional hospitals, district hospitals with development potentials) in Taiwan, TTY will enter the international market through developing high barrier and concept-proved new drugs and biologics with innovative formulations. Presently, TTY has been successful in introducing its own R&D products to regions including EU, Asian Pacific, Middle East regions, Africa, and South America, and has become the best partner for companies which are strong in drug marketing. In the future, TTY will continue its cultivation of Asian target markets (home market) to build up its local strength to become the best partner with the best drug development and marketing capabilities for international innovation and biotech companies in global market. TTY expects to put in low-level investment in developing drugs, biological agents, and innovative drugs of brand new ingredients, highly competitive barrier, and high economic values. By doing so, TTY will create the best profit. It will also establish long-term stable development and share the common good with its international partners.
Milestone
| 1960 | TTY Biopharm Co., Ltd. was established as a traditional generic drug company which focused on production and sales. |
| 1968 | TTY set up a plant in Chungli city, and cooperated with JOZO Co. Ltd. from Japan. |
| 1988 | TTY signed a technical cooperation agreement with pharmaceutical companies, Pierre Fabre from France and Schering-Plough from U.S. |
| 1991 | TTY signed technical cooperation agreement with U.S. pharmaceutical company, Mentholatum, and U.S. based company, Bristol-Myer Squibb. |
| 1993 | Business in Shanghai and China started. A new factory in a joint venture with Shanghai XuDong HaiPu Pharmaceutical Co., Ltd. was set up to develop the Great China market together. |
| 1995 | TTY signed a technical cooperation agreement with Germany-based Knoll AG (now a part of the pharmaceutical giant, Abbott). |
| 1997 | TTY merged with Dong Xing Pharmaceutical Co., Ltd., and total capital reached to NT 180 million. |
| 2000 | A GMP certified plant and an anti-cancer drug plant were approved in Shanghai for providing medical services for Chinese people. |
| 2001 | TTY pharmaceutical plant for orally administered cancer medication in Chungli was completed. TTY launched its IPO in Taiwan OTC biotechnology listing. |
| 2002 | Lipo-Dox, a liposomal injection, won the silver award of 2002 Research Prize of Biomedical Technology from Department of Health, Ministry of Economic Affairs. |
| 2004 | TTY acquired exclusive development rights of S1, an anti-cancer drug, in Taiwan from Taiho in Japan. |
| 2005 | TTY won the Most Innovative Corporation Award of the 13th National Industrial Innovation Award. |
| 2007 | TTY pharmaceutical plant for cancer injection formulation in Chungli was completed. |
| 2008 | TTY pharmaceutical plant for cancer injection formulation in Chungli passed the EU (EMEA) inspection. |
| 2009 | TTY pharmaceutical plant in Neihu passed the FDA inspection for the compliance of PIC/s GMP guideline. TTY pharmaceutical plant for orally administered cancer medication in Chungli passed the EU inspection. TOT Translational Lab acquired ISO17025 certification. Developed liposomal doxorubicin drugs with to-BBB Pharmaceutical Company of Holl and TTY Chungli Factory obtained the Best Medicine Manufacturing Specifications as well as the PIC’s GMP Certification from the Department of Health. |
| 2010 | To spin-off the medical drugs business office and to set up a new TSH Biopharm Co., Ltd. TTY made the acquisition of the Taiwan plant of Shionogi Inc. in Liou-Du. TTY Chungli plant passed the official Arabia inspection (Bahrain, Oman, and Yemen). TTY Neihu plant passed the EMA inspection for the compliance of PIC/s GMP guideline. |
| 2011 | TTY Vietnam office acquired the business license. TTY formulation team acquired the certification of the compliance of PIC/s GMP guideline. The opening ceremony of the reconstruction of the PIC/s GMP plant in Liou-Du was held. Lipo-Dox won the 2011 Symbol of National Quality from Institute for Biotechnology and Medicine Industry. “Process for producing liposome suspensions and products containing liposome suspensions produced thereby” won the silver medal of National Invention and Creation Award from the Intellectual Property Office, Ministry of Economic Affairs. |
| 2012 | TTY Chungli plant passed the official Brazil inspection. Opening ceremony of the TOT pharmaceutical plant for anti-cancer drugs in Suzhou was held. |
| 2013 | TTY Chungli plant passed the official Japan inspection. TTY formulation team established a GMP guideline-compliant excipient plant. TTY won the Outstanding Company of the Year from the Taiwan Bio Industry Organization Award. TTY pharmaceutical plant for orally administered cancer medication in Lioudu passed the TFDA inspection for the compliance of PIC/s GMP guideline. Chungli factory was inspected by US FDA (comply with PIC/s GMP guideline). |
| 2014 | Acquisition of a Taiwanese drug permit license for Brosym for Injection. Neihu Plant passes Taiwan TFDA plant certification. |
| 2015 | Neihu Plant passes Taiwan TFDA PIC/S GMP plant certification. Chungli Factory passes Taiwan TFDA PIC/S GMP plant certification. In order to adjust investment structure, selling all equities of Taiwan Tungyang International Company Limited and TOT Biopharm International Company Limited. |
| 2016 | Audit committee was established to replace supervisor. Lioudu Factory passes Taiwan TFDA PIC/s GMP plant "Lyophilized powder for injection (aseptic preparation) and Injection (aseptic preparation and terminal sterilization)" certification. TTY and the international company will jointly develop the overseas market for the Liposome product. Lioudu factory passed Taiwan TFDA PIC/S GMP inspection and obtained certification in freeze-drying dosage, sterile preparation and final sterilization. The company passed level A inspection of 「Taiwan Intellectual property Management Regulation」. |
| 2017 | Achieved top 5% performance of TPEx-listed companies in the 3rd Company Governance Assessment. TTY and 2-BBB MEDICINES BV set up the Taiwan-based joint venture company EnhanX Biopharm Inc. |
| 2018 | Achieved top 5% performance of TPEx-listed companies in the 4th Company Governance Assessment. TTY Biopharm and global player jointly develop generic drug of Arsenic Trioxide for US and Europe market. The company passed level A inspection of 「Taiwan Intellectual property Management Regulation」. |
| 2019 | Achieved top 5% performance of TPEx-listed companies in the 5th Company Governance Assessment. TTY won the silver award of 2019 (TCSA) Taiwan Corporate Sustainability Awards. |
| 2020 | Achieved top 5% performance of TPEx-listed companies in the 6th Company Governance Assessment. Top 10% of TPEx-listed and OTC companies in non-financial nor electronic stocks with a market value of more than NT$10 billion. Acquired the award of Best Companies to Work for in Asia 2020 Taiwan Edition. TTY won the Traditional Manufacturing silver award of the 2020 TCSA Taiwan Corporate Sustainability Report Category. The company passed level A inspection of 「Taiwan Intellectual property Management Regulation」. |
| 2021 | Achieved top 5% performance of TPEx-listed companies in the 7th Company Governance Assessment. Top 10% of TPEx-listed and OTC companies in non-financial nor electronic stocks with a market value of more than NT$10 billion. TTY won the Platinum Award in the Healthcare Category of the TCSA Taiwan Corporate Sustainability Report. |
| 2022 | Achieved top 5% performance of TPEx-listed companies in the 8th Company Governance Assessment. Top 11%~20% of TPEx-listed and OTC companies in non-financial nor electronic stocks with a market value of more than NT$10 billion. TTY won the Platinum Award in the Healthcare Category of the TCSA Taiwan Corporate Sustainability Report. |
| 2023 | Achieved top 5% performance of TPEx-listed companies in the 9th Company Governance Assessment. Top 11%~20% of TPEx-listed and OTC companies in non-financial nor electronic stocks with a market value of more than NT$10 billion. TTY won the Gold Award in the Healthcare Category 1 (annual revenue NTD$ 5 billion and above) of the TCSA Taiwan Corporate Sustainability Report. TTY was honored with the Best Workplace Award of the 1111 Job Bank, the Taiwan's premier online recruitment platforms. |
| 2024 | TTY won the Best Attractiveness Award in the "2024 Employer Brand Awards" of the 104 Job Bank. TTY won the Gold Award in the Healthcare Category 1 (annual revenue NTD$ 5 billion and above) of the TCSA Taiwan Corporate Sustainability Report. |
| 2025 | Achieved top 5% performance of TPEx-listed companies in the 11th Company Governance Assessment. Top 11%~20% of TPEx-listed and OTC companies in non-financial nor electronic stocks with a market value of more than NT$10 billion. TTY won the Gold Award in the Healthcare Category 1 (annual revenue NTD$ 5 billion and above) of the TCSA Taiwan Corporate Sustainability Report. |